COFEPRIS warns about counterfeit drugs against cancer and types 2 diabetes
The Federal Commission for the Protection against Sanitary Risks (COFEPRIS) issued an alert about the counterfeiting of a drug against breast cancer and the alteration in treatment for type 2 diabetes.
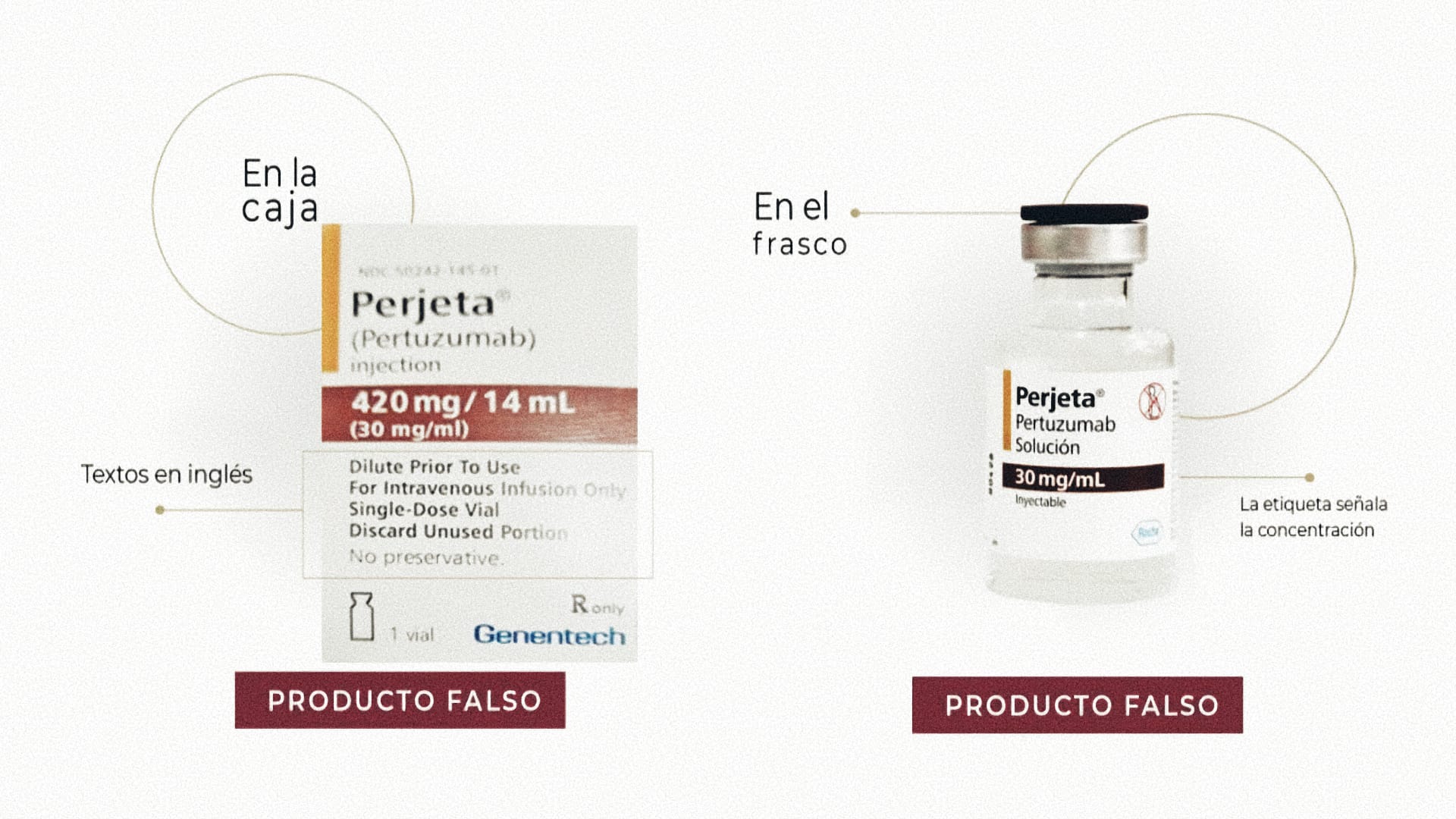
The agency warned medical personnel, pharmacies, and patients that the drug called Perjeta (pertuzumab), indicated for patients with breast cancer, was falsified in the following batches:
- H0343B08
- V4322H37
- H0498B16
All the above are solutions with a concentration of 420mg/14mL (30mg/mL).
COFEPRIS indicated that these drugs put the health of those under treatment with this cancer drug at risk since the agency cannot guarantee good manufacturing practices or the quality and safety of its ingredients.
COFEPRIS reported that the batch number should be carefully reviewed for the population to identify counterfeit products. In addition, the box shows texts very close to the fold line at the top, no legends below the formula, and texts in English; while on the bottle, the label indicates the concentration, as shown in the following images:

On the other hand, COFEPRIS announced the result of technical analysis and the appearance of the holder of the Forxiga health registry, a treatment used for glucose control in patients with type 2 diabetes mellitus.
Given this, the pharmaceutical company AstraZeneca, holder of the Forxiga health registry, pointed out that the alteration was made on the primary and secondary packaging of lot LM0204, due in August 2021; data that was covered with the denomination MJ0017, which has a false expiration date of June 2022.
In primary packaging, you can see scratches in bright black ink that cover expiration date and batch number data, while in the secondary packaging, it presents a box in bright black ink covering original data, batch and expiration date printed in white ink, and two lines, one black and one orange as follows:

For this reason, COFEPRIS recommended acquiring these treatments in pharmacies with a health license. In case of detecting the characteristics indicated, the unit called on the population to present the corresponding health complaint through email pharmacovigilancia@COFEPRIS.gob.mx.
Source: El Financiero
The major issues for the further development of the pharmaceutical market will be discussed at the Mexican Pharmaceutical Forum that will be organized by the Global Pharmaceutical Leaders’ Club on the 29-30th November 2022 in Mexico City. Secure your delegate place ASAP! Registration is here: https://mexicanpharmaforum.com/registration/
Recommended Posts

Takeda appoints Hernán Porcile as its new General Manager in Mexico
October 24, 2022
Solid growth of the Medical Devices sector in Mexico
October 19, 2022

Sandoz Receives Certification for Transparent Practices
October 19, 2022
